What is an organoid and why use it in research?
There has been an increasing shift towards the development of 3D cell culture models in attempts to create an increased complexity that can be compared with the in vivo better than 2D models. From the different 3D models, cell aggregates are particularly interesting in mimicking organs and tumours because of the close cell-cell interactions in multiple planes, self-organization and gene and protein expression they reflect.
Cell aggregates can be classified as spheroids or organoids. Both come from the assembly of cells compactly in a 3D structure but differ in the type of cells that compose the aggregate. Spheroids are composed of cells from commercial cell lines, single cells or cells collected from tissues. These cells organise themselves as solid tumours would, with a proliferative, a quiescent and a hypoxic region. This structure similarity between tumours and spheroids is translated into a similar response to chemotherapeutics which is quite advantageous in drug screening assays to better predict their outcome and more efficiently move to in vivo studies. The spheroids can also be made of different cell types, forming a multicellular spheroid.
Organoids are formed from stem cells that differentiate into specific cell types and self-organize. It is common to use a matrix to embed organoids and facilitate the maintenance of this self-organization of the cells. There might be confusion between multicellular spheroids and organoids but while multicellular spheroids present a disorganized proliferation from a cell cluster, organoids are organized and polarized structures, including lumens in organoids such as gastrointestinal and lung organoids. The presence of polarized cells and lumen in organoids gives access to important studies (from cell-cell interaction to drug behaviour) that 2D models are unable to replicate.
So far more than 15 different types of organoids have been generated to study organs such as the brain, liver, kidney or intestine in fields such as embryogenesis, organogenesis, pathogenesis, drug discovery or personalized medicine. Some organoids even demonstrated specific organ functions such as neural activity in brain organoids or periodic contraction in cardiac organoids.
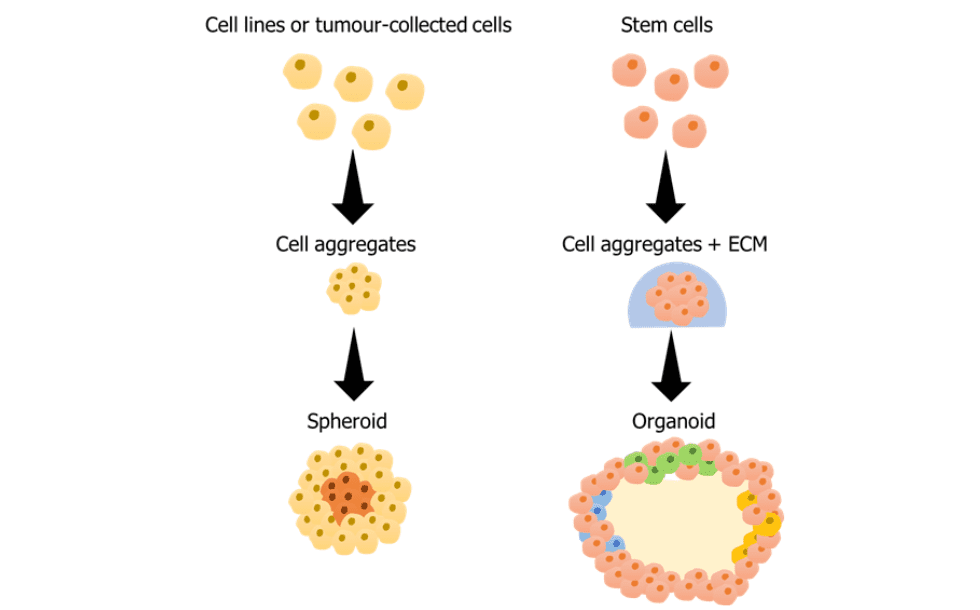
Figure 1 Scheme of spheroid and organoid production. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
In most diseases, it is known that not all patients respond equally to the existing treatments which affects the effectiveness of the drug. Spheroids and organoids can be produced from patient cells and differentiate into healthy or diseased models that better reflect the patient. This is advantageous when choosing which treatment would be more efficient for a patient, increasing the survival rate and allowing a more personalized medicine7.
Even though organoids are interesting and useful cell culture models, they still present some disadvantages8. Most organoid protocols take several weeks to reach a mature state where different assays can be performed. During this time, the organoids increase in size up to a few millimetres which is accompanied by the difficulty in medium diffusion creating a hypoxic centre. Models such as tumour spheroids can take advantage of the hypoxic core since it is also present in human tumours, however, healthy organs do not possess this feature and the presence of it in organoid models does not reflect the conditions of the organ that is intended to mimic.
Why combine organoids and microfluidic devices?
1. Decrease of hypoxia conditions in the centre of the organoid
Microfluidic devices are compartmentalized platforms capable of introducing architecture and mechanical cues to cell culture. These models are particularly interesting to be coupled with organoid culture. One of the most interesting characteristics of organoids-on-a-chip is the exposure of the culture to continuous fluid flow, which increases the diffusion of cell media and decreases hypoxia on the organoid core. The inclusion of a perfusion system also automates the system by decreasing the need for human manipulation of organoid culture, especially the need to frequently change the medium manually.
2. Introduction biomechanical and biochemical force stimuli
The use of flow in culture is not only advantageous to avoid hypoxic conditions but also to introduce shear forces and gradients. The exposure of these forces influences biological processes such as the activation of mechano-proteins and improves the vascularization of organoids. Apart from vascularization, other mechanical forces are important in the development and maturation of organs. The biomechanical control of these forces is very difficult to achieve in 2D or 3D models, but microfluidic devices are being developed to be mechanically active and apply these forces to organoid culture.
In the organogenesis process, the different layers and tissue organization is dependent on exposure to different stimulus at different time points. In 2D cultures, it is difficult to expose the cells to different stimuli (chemical, physical, etc.) in the same well. Microfluidic devices, with their different compartments and channels, can create physical stimulus in only a side of the culture or a chemical gradient. This feature allows greater control over the differentiation of the organoid into a mature state to better mimic the native organ. The generation of chemical gradients through microfluidic devices is also interesting in drug screening (Figure 3 A). With a combination of channels and well, spheroids or organoids can be cultured in a high number and exposed to different concentrations of drugs, all in one assay.

Figure 2 Summary of advantages of combining microfluidic devices with organoid culture.
3. Production of homogeneous replicates
Organoid production is often linked with the generation of heterogeneous replicas. This can be a disadvantage since different-sized organoids cannot be considered triplicates in experiments. To aid the production of organoids, microfluidic devices capable of generating droplets can be used (Figure 3 B). Using droplet microfluidics to produce organoids allows the generation of homogeneous replicas at a fast pace, permitting the high throughput production of these models.
Microfluidic devices can act not only as homogeneous organoid generators but also as a filter to separate organoids with different sizes and allow the collection of similar-size replicas (Figure 3 C).
4. Use of smaller volumes and decreasing expenses
Stem cell differentiation protocols are usually associated with long-term cultures and expensive cell culture media. Organoid culture is also associated with these characteristics. The pairing of organoid culture and microfluidic platforms can decrease the expenses with media culture since microfluidic devices use a smaller volume of liquid to nourish the cultured cells.
5. High-throughput production and maintenance
For drug screening assays, the ability to perform high throughput culture and analysis is very interesting, especially from an industry point of view. As mentioned above, droplet microfluidic platforms can produce homogeneous and high-quantity organoids. Besides this massive production, microfluidic devices are also able to serve as a culture and maintenance platform for a high number of organoids, such as a microwell array (Figure 3 D). Depending on the design, this platform can act as only maintenance where all organoids are exposed to the same cultured media or organoids can be seeded in different compartments or channels and exposed to different media or different concentrations of media/substances.

Figure 3 Examples of microfluidic platforms for spheroid/organoid culture. (A) Microfluidic platform for the generation of chemical gradients for photothermal therapy in spheroids. Figure reprinted with permission from. (B) Platform for the generation of organoids through droplet microfluidics. Image reprinted with permission from. (C) Microfluidic device capable of spheroid trapping and filtration. Image reprinted with permission from. (D) Microfluidic platform for the co-culture of several replicas of liver and islet organoids. Image reprinted with permission from. (E) Compartmentalized device for the co-culture of kidney organoids and endothelial cells. Image reprinted with permission from. (F) Sensors coupled with a microfluidic device for 3D culture. Image reprinted with permission from.
6. Co-culture
The compartmentalization of microfluidic platforms is useful not only to host and organize organoids but also to introduce other cell types in the cell culture models. One of the most interesting cell types to co-culture with organoids is endothelial cells (Figure 3 E). To fully support long-term culture and better mimic tissues, the presence of a vascularization network integrated with the organoid is an important feature that several studies intend to create with the co-culture of endothelial cells. On another note, the embryogenesis process is also coupled with the vasculature development which means that a vascularized organoid would be a more accurate replicate of the organogenesis. Creating a vascularized organoid is not easy for all types of organoids. While in tumour organoids the co-culture of tumour cells and endothelial cells can be achieved in vitro, the same cannot be easily translated into an organoid derived from pluripotent stem cells. iPSC differentiation protocols include a variety of different small molecules that are introduced in the culture in different concentrations according to specific time points. The inclusion of endothelial cells in the culture including its medium can change the expected outcome of the iPSC differentiation. However, microfluidic devices can still help IPSC-derived organoids avoid a necrotic centre by acting as mini-bioreactors, increasing the media diffusion through the organoid.
Another interesting cell type to include in organoid models is immune cells. Particularly in tumour models, the study of the reaction of immune cells to the tumour itself and tumour treatments can bring important insights into the tumour response to therapy.
Apart from culturing different cell types in the same microfluidic device and/or organoid, different microfluidic platforms or compartments can be connected through media flow. This allows the culture of organoids from different tissues to form a multiorgan system. This system can be used to study the interaction of the different organs with themselves or to test drugs and evaluate the side effects of these compounds in tissues/organs that the drug was not designed to act on.
7. Integration of sensors
Apart from the production and culture/maintenance of organoids and spheroids, microfluidic platforms can be an interesting choice for the analysis of these models. Most microfluidic devices are fabricated with transparent materials which allow the monitoring of the culture through microscopy techniques, during culture or/and after staining. Besides imaging techniques, sensors can be added to the platform to monitor culture parameters such as oxygen levels, pH or temperature sensors (Figure 3 F)24,30. This monitoring can be useful to ensure the culture is progressing well and to monitor the cell growth, viability, metabolic activity, and maturation of the cells. Cardiomyocytes and neural cells present electrophysiological activity when reach a mature state or phenotype. The coupling of multi-electrode arrays into microfluidic platforms helps monitor the activity levels of the cells without stopping the culture31. This translates into a less manually handled culture and a smaller number of replicates for different time points of activity monitoring.
References
- Hofer, M. & Lutolf, M. P. Engineering organoids. Nat Rev Mater 6, 402–420 (2021).
- Huang, B.-W. & Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. Journal of Controlled Release 270, 246–259 (2018).
- Velasco, V., Shariati, S. A. & Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst Nanoeng 6, 76 (2020).
- Fang, G., Chen, Y.-C., Lu, H. & Jin, D. Advances in Spheroids and Organoids on a Chip. Adv Funct Mater 33, 2215043 (2023).
- Sharf, T. et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat Commun 13, 4403 (2022).
- Kim, H., Kamm, R. D., Vunjak-Novakovic, G. & Wu, J. C. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 29, 503–514 (2022).
- Nounsi, A. et al. Patient-Derived Tumoroid for the Prediction of Radiotherapy and Chemotherapy Responses in Non-Small-Cell Lung Cancer. Biomedicines 11, 1824 (2023).
- Zhao, Z. et al. Organoids. Nature Reviews Methods Primers 2, 94 (2022).
- Cho, A.-N. et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun 12, 4730 (2021).
- Lee, H. N. et al. Effect of biochemical and biomechanical factors on vascularization of kidney organoid-on-a-chip. Nano Converg 8, 35 (2021).
- Fang, G. et al. Enabling peristalsis of human colon tumor organoids on microfluidic chips. Biofabrication 14, 015006 (2022).
- Charelli, L. E., Ferreira, J. P. D., Naveira-Cotta, C. P. & Balbino, T. A. Engineering mechanobiology through organoids-on-chip: A strategy to boost therapeutics. J Tissue Eng Regen Med 15, 883–899 (2021).
- Rifes, P. et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat Biotechnol 38, 1265–1273 (2020).
- Prince, E. et al. Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies. Adv Healthc Mater 11, 2101085 (2022).
- Lim, W. & Park, S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules 23, 3355 (2018).
- Wang, Y., Liu, M., Zhang, Y., Liu, H. & Han, L. Recent methods of droplet microfluidics and their applications in spheroids and organoids. Lab Chip 23, 1080–1096 (2023).
- Lee, J. M. et al. Generation of tumor spheroids using a droplet-based microfluidic device for photothermal therapy. Microsyst Nanoeng 6, 52 (2020).
- Jin, B.-J. et al. Microfluidics platform for measurement of volume changes in immobilized intestinal enteroids. Biomicrofluidics 8, (2014).
- Bourn, M. D. et al. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. Journal of Controlled Release 326, 13–24 (2020).
- Zhu, Y. et al. In situ generation of human brain organoids on a micropillar array. Lab Chip 17, 2941–2950 (2017).
- Tao, T. et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19, 948–958 (2019).
- Tao, T. et al. Microengineered multi‐organoid system from hiPSCs to recapitulate human liver‐islet axis in normal and type 2 diabetes. Advanced Science 9, 2103495 (2022).
- Bas-Cristóbal Menéndez, A. et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci Rep 12, 20699 (2022).
- Dornhof, J. et al. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 22, 225–239 (2022).
- Schulla, L. S. et al. Development of a Novel Microfluidic Co-culture model to study Organoid Vascularization. bioRxiv 2022.03.25.485813 (2022) doi:10.1101/2022.03.25.485813.
- Berger, E. et al. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 18, 3172–3183 (2018).
- Zhang, J. et al. Immunotherapy discovery on tumor organoid-on-a-chip platforms that recapitulate the tumor microenvironment. Adv Drug Deliv Rev 187, 114365 (2022).
- Skardal, A. et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7, 8837 (2017).
- Koning, J. J. et al. A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin. Frontiers in Toxicology 3, (2022).
- Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceedings of the National Academy of Sciences 114, E2293–E2302 (2017).
- Spitz, S. et al. Development of a multi-sensor integrated midbrain organoid-on-a-chip platform for studying Parkinson’s disease. bioRxiv 2022.08.19.504522 (2022) doi:10.1101/2022.08.19.504522.

